

We calculate that they exist in cloud-like orbits around the nucleus of the atom. Sometimes they can even leave the atom if they find something more attractive. Electrons are negative, have almost no mass, and are located on the outer margins of the atom and are in constant motion around the nucleus, like bees around the mouth of a hive.What part of atoms is not stable?Ītoms have three basic types of subatomic particles: electrons, protons, and neutrons. For this reason, these two groups are often found with each other as ionic substances and with most other elements. These two groups have an electron to donate or have room to gain one electron from another atom. On the other end of the spectrum, for example, are the alkali metals and the halogens, two groups that are extremely reactive, almost never existing in their pure elemental forms in nature. Outside of these noble gases, different elemental groups vary in how stable they are in their pure forms, or how easily they form compounds with other elements. For this reason, they are called inert and are most stable in their pure elemental forms.
#Helium valence electrons full
These elements have full electron configurations in their outermost energy level orbits (rule of octet again), and hence no potential for picking up or sharing electrons belonging to other elements.
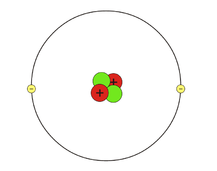
These elements at the end of each row are so stable that it is difficult to make them react under any conditions. An interesting exception can be found in group 8A containing helium, neon, argon and the other noble gases. Most elements don’t exist in their pure forms as they are depicted on the table because they are more stable when reacted with other elements and substances. The periodic table is arranged in such a way to help us predict reactions based on atomic structure and the shared characteristics of groups of elements that share a set number of valence electrons. StabilityĪtoms tend to arrange themselves in a way that is most stable, or at the lowest energy. We have reviewed the essential nature of electron orbits and their role in determining many of the fundamental differences between the classes and groups of elements and how they interact. Now we will turn to the atomic structure, an atom’s stability, and one of the best ways to show electron distribution, with a Lewis dot structural diagram. ⬅ Previous Lesson Workshop Index Next Lesson ➡


 0 kommentar(er)
0 kommentar(er)
